Patient Testimonials
 |  |
| Damaris Olagundoye, MD, grew up knowing that her maternal grandmother was a breast cancer survivor. She would listen as her mother talked with cousins who had also been diagnosed with the disease, but her family didn’t learn until decades later that they carried a mutation in the BRCA2 gene. Researchers at Vanderbilt-Ingram Cancer Center, where Olagundoye is a patient, are putting Black women front and center as they investigate why breast cancer is deadlier in women of African ancestry than other racial groups. Click below to read more about Dr. Olagundoye. https://momentum.vicc.org/2022/11/hidden-risks/ | Kelsey Brauman, Courtni Hammers, and Sam Shreve are survivors of a rare cancer, who met in VICC clinic. All three women are now post-treatment and on a schedule of routine monitoring to spot any potential cancer recurrence for a minimum of 10 years. During that time, they know one thing for sure. The bond they formed due to their diagnoses will remain. They plan to continue texting, calling, checking in via Facebook posts and even meeting up when they can. Click below to read about their individual stories and collective bond. https://momentum.vicc.org/2022/04/sarcoma-sisterhood/ |
Participation
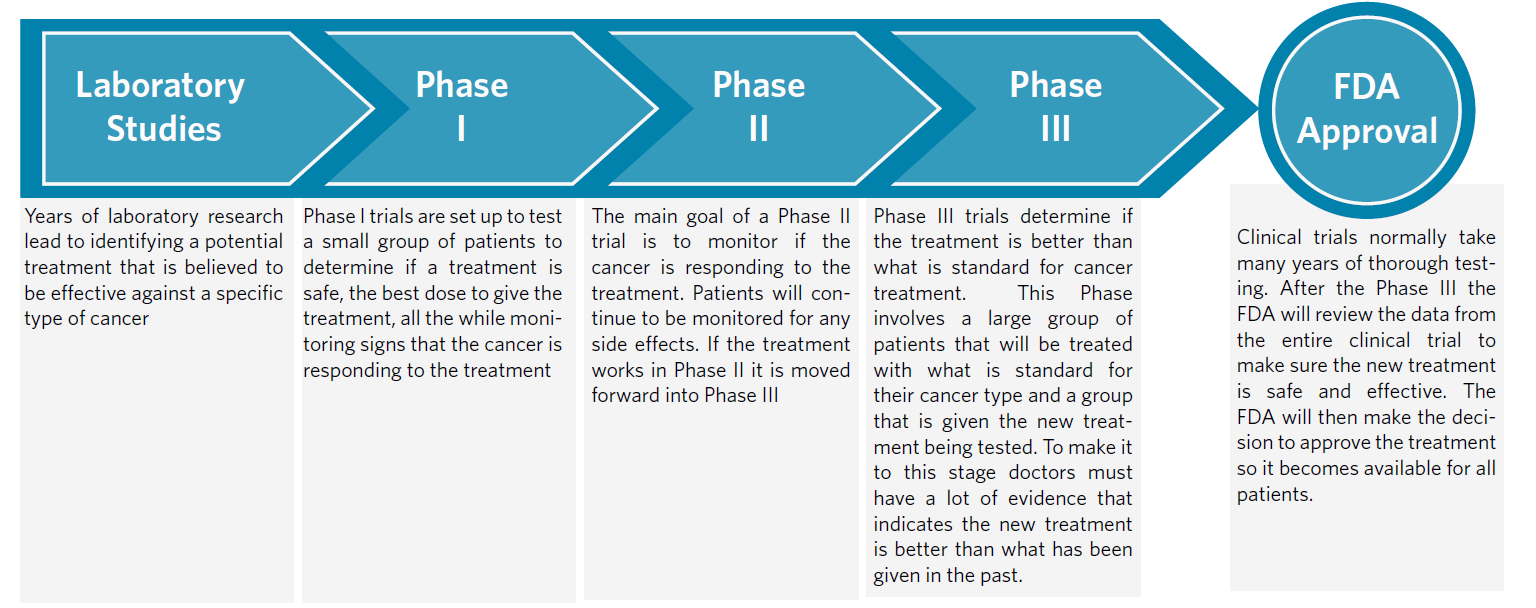
As a volunteer, you have the opportunity to be involved in clinical research that may bring about advances in science and healthcare. There are risks that you need to know about before you decide to enroll in a clinical trial. Any questions you may have about the study will be answered before you start, but before a new treatment is tested with patients it is carefully studied for several years in a laboratory and tested for safety. At Vanderbilt, clinical trials undergo a rigorous approval process both by the U.S. Food and Drug Administration (FDA) and by two separate committees at the Medical Center. Patients who qualify for a trial decide, with advice from their doctor, whether to participate in a study. The patient will learn about both the risks and benefits of the study before making a decision.
Phases of a Clinical Trial
 |
 |
Meet our Physicians
 |
 |
| Fall 2022- "Vanderbilt-Ingram Cancer Center (VICC) physicians are leading two landmark clinical trials that have the potential to greatly expand care options for people with colorectal cancer. One offers a new option for metastatic colorectal cancer that has not responded to existing treatments. The other could bring about a paradigm shift in how rectal cancer is treated among a subset of patients. Click below to read more about these exciting new therapies" https://momentum.vicc.org/2022/11/breakthrough-therapies/ |
Spring 2022- "Ryan Merrell, MD joined the Vanderbilt faculty in August 2021 as assistant professor of Neurology and director of the Division of Neuro-Oncology, leading the charge to grow Vanderbilt University Medical Center’s nationally recognized brain tumor program. Vanderbilt’s Division of Neuro-Oncology specializes in the diagnosis and management of primary or secondary cancers affecting the brain and the neurologic complications of cancer. Click Here to read the article about Dr. Merrell" https://momentum.vicc.org/2022/04/empathetic-mindset/ |
Frequently Asked Questions
What is the purpose of a clinical research?
Scientists are constantly working to improve diagnosis and treatment of diseases. Clinical trials are necessary to determine if the new discoveries made in a laboratory will translate to improved lives of patients through new treatment options.
Why would I choose to go on a clinical trial?
The decision to join a clinical trial is a personal decision that can be made after talking about your individual diagnosis with your oncologist.
Clinical trials offer access to cutting edge treatments, as our understanding of cancer and the best ways to treat it is constantly being updated. Research is constantly ongoing and updating to new approaches and ways to manage cancer, which leads to more and more clinical trials.
There is a special team of doctors, nurses and other cancer specialists that will be added to your team of care providers that will be available to you for any questions you have before and during the treatment.
Clinical trials provide an excellent opportunity for you to contribute to science and participate in the process of discovery. The treatments patients receive for their cancers all started as clinical trials, and patients who have agreed to participate in those trials have led to improved therapies for others with cancer diagnoses.
What are downsides to joining a clinical trial?
Many trials require increased numbers of imaging and in-person appointments to monitor the progress of the cancer after the new treatment. Some trials will require more blood to be drawn or biopsies to be taken. Some trials may require more visits and, while closer monitoring can be beneficial, it may require a little more time spent in our clinic.
How can I find opportunities to participate in clinical research?
Many times, the place to start is discussing options with your healthcare provider and cancer care team. The volume of clinical trials available to patients is very large and can be difficult to navigate on your own. Vanderbilt-Ingram Cancer Center has a web page you can use to look at your specific cancer and clinical trials available. You can also learn about trials available across the United States by searching the clinical trials website (www.clinicaltrials.gov).
There are factors in trials that sometimes will allow for you to be included, and other factors that may exclude you from joining. Your cancer care team will assist in making sure which trials apply to you.
How much does it cost?
It is smart to ask about cost of joining a trial. Normally, the pharmaceutical companies and other funding agencies pay for the costs associated with the new treatment such as biopsies, lab tests and genetic testing. Insurance will still be responsible to pay for the standard of care treatment which will include the approved treatment, office visits, routine tests, imaging and hospital admissions for urgent problems.
How long does treatment last? How often do I need to be here?
In general, treatment is continued as long as it is helping and does not have too many side effects. Before a trial can begin, a schedule of treatments, visits and scans is approved. Each trial is slightly different, the length of time and treatment pattern will be communicated clearly with patients before they agree to join a clinical trial.
What are side effects and risks?
Side effects and risks will vary depending on each individual trial. Drugs that are just beginning to be studied may have less known about potential side effects. If you choose to take part in a clinical trial, your cancer care team will provide details about what is known about potential risks and provide methods to contact them in case of any side effects.
What does it mean to give informed consent?
Before you start a clinical trial, you will be provided written information regarding the trial you are interested in enrolling. That information will enable you to make the decision about what is best for you in the treatment of your cancer, and then you will be asked to sign the consent to join the study.
Should I be scared to get the placebo?
The vast majority of clinical trials do not involve a placebo. If a placebo is involved, that is clearly communicated to you and it will be explained why that is the case.


